Ultrasound Case Study
Patellar tendinopathy and diagnostic ultrasound
Stuart Wildman, Extended Scope Physiotherapist and MSK Sonographer
This is a case study/overview of patellar tendinopathy and diagnostic ultrasound, focussing on summarising the role of ultrasound in the diagnosis. It aims to provide a source of evidence based information for clinicians. The treatment of tendinopathy and the current thoughts on the mechanisms behind it are outside the scope of the aim of this article.Please feel free to comment at the end of the post to discuss this somewhat debateable area of MSK medicine and musculoskeletal ultrasound.
This patient in his late 20’s presented with an eight month history of right anterior knee pain. This had started insidiously, and had not resolved completely with a course of Physiotherapy. He had found some benefit to performing an eccentric training program, but on return to football his symptoms recurred. On examination, there was no visible effusion of the knee, and he presented with full range of movement. There was some subtle tenderness on palpation of the proximal insertion of the patella tendon. Functional testing was unremarkable, with no pain on eccentric loading.
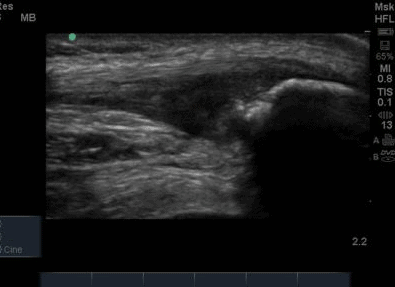
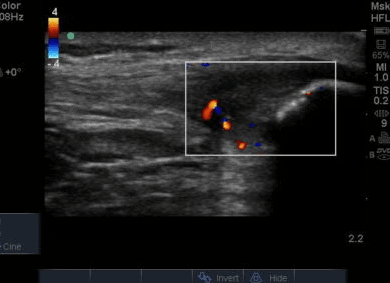
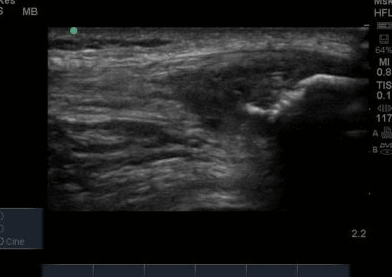
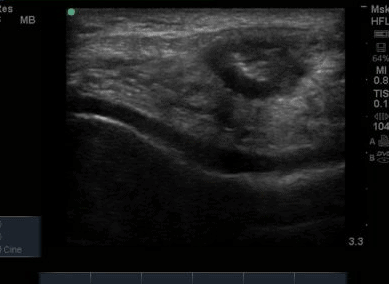
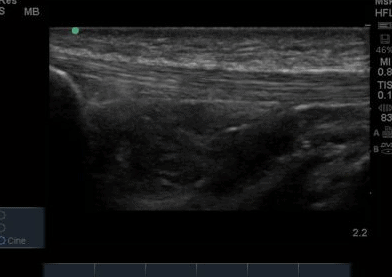
The ultrasound images revealed that the patient had a thickened and severely hypoechoic proximal insertion of the patellar tendon on the inferior pole of the patella. There was a particularly hypoechoic region of the deep proximal fibres as they attached to the patella (Figure 1 and 3). There was a region of cortical irregularity on the cortex of the patellar and also a region of hyperechoic signal within the proximal patella tendon (Figure 1,3 and 4). There was no associated acoustic shadowing, so it was felt this was likely to represent a calcific deposit. There was also marked neovascularity, particularly in the region of the deep proximal fibres attaching to the patella.
‘Severely thickened and hypoechoic patellar tendon consistent with tendinopathic change in both longitudinal and transverse views. Region of hyperechoic signal in the deep proximal aspect of the tendon, and some associated cortical irregularity suggestive of a calcific deposit. Marked neovascularity also present. In summary, these findings are consistent with severe patellar tendinopathy’
Following the ultrasound examination, there was some concern that the cortical irregularity and hyperechoic region within the tendon may represent a bony injury or avulsion from the patella. However, deep to the hyperechoic area there is no significant acoustic shadowing, which you would expect with an area of high acoustic mismatch such as soft tissue and bone. It was therefore felt this was most likely a calcific deposit in keeping with the tendinopathic change and is a common finding in chronic patellar tendinopathy (Comin et al, 2013).
Anterior knee pain is a common cause of referral to musculoskeletal clinics and has been known to make upto 25% of referrals (Christian et al, 2006) and be present in 14% of elite athletes (Lian et al, 2005). There are multiple conditions that can cause anterior knee pain due to their close proximity to each other, including deep infrapatellar bursitis, Hoffas fat pad irritation, patellofemoral joint pain and the synovial plica (Christian et al, 2006). Differentiating these conditions during a clinical examination is often challenging. Subjective information, particularly specific aggravating factors, does offer further clues as to the likely diagnosis (Warden & Brukner,2003). There does however still remain some uncertainty on the ability to differentiate some pathologies and it is common to incorporate grey-scale ultrasonography and Magnetic Resonance Imaging (MRI) into the clinical reasoning process (Warden et al, 2007).
It is often possible for more than one condition to co-exist and that is often where imaging is helpful to offer greater clarity on diagnosis. Diagnosis of patellar tendinopathy is predominantly a clinical diagnosis (Warden et al,2007). As mentioned comprehensive clinical history and examination often provide clues as to the likely structures at fault (Christian et al, 2006; Peace et al, 2006). Localised tendon pain with loading, tenderness to palpation and impaired function are the key clinical signs of tendinopathy (Rio et al, 2013; Warden & Brukner,2003, Christian et al,2006).
Patellar tendinopathy is a degenerative disease of the tendon which leads to anterior knee pain and focal tenderness at the inferior pole of the patella (Dragoo et al, 2014).and is common in a sporting population. It often involves sudden maximal contractions such as lunging and is commonly termed Jumpers knee (Couppe et al, 2013). Tendinopathy is a term that has been recently advocated to describe the variety of painful conditions that develop in and around tendons following unaccustomed or overuse activities. Histopathologic changes associated with tendinopathy include degeneration and disorganization of collagen fibres, increased cellularity, and minimal inflammation (Berg et al, 2013). Patients can also develop macroscopic changes including tendon thickening and loss of mechanical properties, resulting tendon degeneration (Brett et al, 2008).
The patella insertion is commonly the source of symptoms particularly the deeper fibres (Kavanaugh & Yu, 2000). Tendons respond to the strains placed upon them during exercise by increasing cellular activity (Bojsen-Miller et al, 2006) and collagen synthesis (Langberg et al, 2001) both resulting in increased tendon size (Couppe et al, 2013) which can be easily appreciated on imaging. When interpreting an ultrasound image an appreciation of normal sonographic findings is required. The patellar tendon is composed of fascicles of collagen fibers that are distributed in the direction of the force in the long axis (Hodgson et al, 2012) which are bright hyperechoic bands (Lento & Primack, 2008) with a fibrillar echotexture (Jacobsen, 2009). A hypoechoic structure surrounding the tendon will also be present, representing the paratenon (Hodgson et al, 2012). Musculoskeletal ultrasound can be used to visualise structural changes in tendons with characteristic findings of patellar tendinopathy including hypoechoic regions, tendon thickening, calcification and neovascularisation (Hodgson et al,2012).All of these findings were present in the case study that is illustrated in Figure 1-4. Areas of hypoechoic appearance need to be interrogated thoroughly to ensure that the alteration in echotexture is not due to Anisotropy, a commonly encountered artefact of ultrasound (Peace et al, 2006).
Ultrasound is operator and equipment dependent (Christian et al, 2006), and this plays a key role in the quality of images obtained. Black et al (2004) did however demonstrate high intratester reliability of patella tendon sonographic assessments between two MSK radiologists. A limitation of this study was the small sample size. Ultrasound provides an easily accessible and cheap way of confirming the diagnosis. The patellar tendon is easily visualised with the knee partially flexed. As the patellar tendon is a superficial structure a high frequency probe is required to obtain the optimal image (Hodgson et al, 2012). An advantage of ultrasound over MRI is its ability to be used dynamically, allowing visualisation of the tendon from different angles and whilst under stretch or contraction (Hodgson et al, 2012). A disadvantage of ultrasound compared to MRI is its need for an acoustic window, and its inability to visualise past bone (Hodgson et al, 2012). On MRI, patellar tendinopathy is characterized by a focal increase in signal intensity, seen first on gradient echo images, followed by T1 weighted spin echo images(Hodgson et al, 2012). There is also likely to be a change in thickness as discussed with ultrasound.
Ultrasound has been found to superior to MRI in diagnosing clinically confirmed patellar tendinopathy (Warden et al, 2007) with higher specificity and sensitivity. Thirty patients with clinically diagnosed symptoms were investigated and compared with an asymptomatic group. A good feature of the methodology was that the radiologists were blinded to the clinical history and MRI results improving the validity of the study.
The development of vascularity in a normally avascular structure has been commented on as being related to tendon pathology, and is often termed neovascularisation. This has been a significant area of research in both patellar and achilles tendon research in recent years. Neovascularization is the increase in microcirculatory blood flow (Divani et al, 2012) and the ingrowth of vessels is thought to be accompanied by nerves and often proposed to be related to the development of tendon pain. Patients with neovascularity of patellar tendons have higher pain levels with tendon loading and poorer functional outcomes (McCreesh et al, 2013) however it has also been found to be a common finding in asymptomatic patellar tendons as well (Comin et al,2013). This conflicting research is mirrored in chronic achilles tendon disorders where Ohberg et al (2001) reported small blood vessel ingrowth in all symptomatic chronic achilles tendon disorders, and Peers et al (2003) found a positive correlation between neovascularization and visual analogue scale. However other studies have reported that between 47-88% of symptomatic tendons have neovascularization. (Peers et al, 2003; Zanetti et al, 2003, De Vos et al, 2007). These varied outcomes may in part be due to the difficulty to clarify the degree of neovascularization and grade it in research. There has been no research comparing the site of patellar tendon tenderness with areas of neovascularity, but it has been found to correlate in achilles tendons (Divani et al, 2010). The use of Colour and Doppler flow is however of value, and offers a significant advantage over MRI imaging (Malliaras et al,2010).
Although both ultrasound and MRI are useful, they cannot be considered a gold standard for the diagnosis of patellar tendinopathy in isolation. There is a well reported high incidence of sonographic findings such as diffuse thickening, increased vascularity and focal abnormalities in high-level sporting populations (Comin et al, 2013; Cook et al, 2001). It is important to acknowledge that these sonographic appearances are not always associated with pain, meaning correlation of imaging findings and tissue pathology is not always possible (Kulig et al, 2013). This calls into question the clinical utility of abnormal sonographic findings (Kulig et al, 2013). Comin et al (2013) carried out a cohort study with a 24 month follow up on ballet dancers (79 tendons). They found a weak association between the presence of moderate-to-severe hypoechoic defects at baseline and the development of symptoms and no evidence that any of the sonographic findings predicted a problem in the future. Further questioning the impact of sonographic findings on patient management.
It is likely that the choice on whether to utilise MRI or ultrasound is part based on the ability of the clinician to access either modality. With an awareness of the limitation of correlating symptoms and imaging findings, it is likely that clinicians will utilise imaging not to confirm a diagnosis but to rule out other coexisting pathology and assist the reasoning process. Ultrasound is well placed as an adjunct to a clinical examination, and not a gold standard in isolation to dictate the choice of intervention or prognosis (Warden et al, 2007). Certainly, MRI and Ultrasound complement each other as both have unique features which assist forming a diagnosis.
Access to ultrasound in clinic confirmed the clinical suspicion, and also clarified the lack of other co-existing pathology in the symptomatic region. If used appropriately, ultrasound should not be used at face value to provide a quick diagnosis dependent on the sonographic findings, as the current research does not support this approach. It should be integrated carefully into the clinical reasoning process, where ultrasound findings will be carefully explained and placed in the correct clinical context.
References
Berg, K. Peck. J, Boulger, C. & Bahner, D. (2013) ‘Patella tendon rupture an Ultrasound
case report’, British Medical Journal Case Studies.
Black, J. Cook, J. Kiss, Z. S. and Smith, M. (2004) ‘Intertester reliability of sonography in
patellar tendinopathy’, Journal of Ultrasound in Medicine, 23(5),pp.671-675.
Bojsen-Møller J, Kalliokoski KK, Seppanen M, Kjaer M & Magnusson SP (2006)
‘Low-intensity tensile loading increases intratendinous glucose uptake in the Achilles
tendon’, Journal of Applied Physiology, 101, pp.196–201.
Brett, M. Andres, M. D. George, A. C. & Murrell, M. D. (2008) ‘Treatment of Tendinopathy:
What works, what does not and what is on the horizon?’, Clinical Orthopaedic Related
Research, 466, pp.1539-1554.
Christian, S. R. Anderson, B. Workman, R. Conway, W. F. & Pope, T. L. (2006) ‘Imaging of
Anterior Knee Pain’, Clinics of Sports Medicine, 16 (1), pp. 681-702.
Cook, J.L, Khan, K.M., Kiss, Z.S., Coleman, B.D., Griffiths,L. (2001) ‘Asymptomatic
hypoechoic regions on patellar tendon ultrasound: A 4-year clinical and ultrasound followup
of 46 tendons’, Scandinavian Journal of Science and Medicine in Sports, 11, pp.321-327.
Comin, J. Cook, J. L. Malliaras, P. et al. (2013) ‘The prevalence and clinical significance of
sonographic tendon abnormalities in asymptomatic ballet dancers: a 24-month longitudinal
study’, British Journal of Sports Medicine, 47, pp.89-92.
Couppe C, Kongsgaard M, Aagaard P, Vinther A, Boesen M, Kjaer M,Magnusson SP.
(2013) ‘Differences in tendon properties in elite badminton players with or without patellar
tendinopathy’, Scandinavian Journal of Medicine and Science in Sports, 23, e89–e95.
De Vos, R.J., Weir, A., Cobben, L.P., Tol, J.L. (2007) ‘The value of power Doppler
ultrasonography in Achilles tendinopathy: a prospective study’, American Journal of Sports
Medicine, 35,pp.1696-1701.
Divani, K., Chan, O., Padhiar, N., Twycross-Lewis, R., Maffulli, N., Crisp, T., Morrissey, D.
(2010) ‘Site of maximum neovascularisation correlates with site of pain in recalcitrant mid-
tendon Achilles Tendinopathy’, Manual Therapy,15(5), pp.463-6.
Dragoo J.L, Wasterlain AS, Braun HJ, Nead KT.(2014) ‘Platelet-rich plasma as a treatment
for patellar tendinopathy: a double-blind, randomized controlled trial’, American Journal of
Sports Medicine, 42(3), pp. 610-8.
Hodgson, R. J. Grainger, A. J. O’Connor, P. J. Evans, R. Coates, L. Marzo-Ortega, H. et
al.(2011) ‘Imaging of the Achilles tendon in spondyloarthritis: a comparison of ultrasound
and conventional, short and ultrashort echo time MRI with and without intravenous contrast’,
European Radiology, 21,pp.1144–52.
Hodgson, R. J. O’Connor, P. J. Grainger, A. J. (2012) ‘Tendon and ligament imaging’, British
Journal of Radiology,85, pp.1157-1172.
Jacobson, J. A. (2009) ‘Musculoskeletal Ultrasound: Focussed Impact on MRI’, American
Journal of Roentgenology,193, pp.619-627.
Kavanaugh.J. & Yu, J.S.(2000) ‘Too much of a good thing: overuse injuries of the knee’,
Magnetic Resonance Imaging Clinics of North America, 17(4),pp.725-739.
Kulig, K., Landel, R., Chang, Y-J., Hannanvash, N.,Reischl,S.F., Song, P. & Bashford,
S.R. (2013) ‘Patellar tendon morphology in volleyball athletes with and without patellar
tendinopathy’,Scandinavian Journal of Medicine and Science in Sports, 23, e81-e88.
Lian, O. B. Engebretsen, L. & Bahr, R. (2005) ‘Prevalence of jumper’s knee among elite
athletes from different sports: a cross-sectional study’, American Journal of Sports Medicine
33, pp.561-567.
Lento, P. H. & Primack, S. (2008) ‘Advances and utility of diagnostic ultrasound in
musculoskeletal medicine’,Current Review of Musculoskeletal Medicine, 1, pp.24-31.
Malliaras, P. Purdam, C. Maffulli, N. & Cook, J. (2010) ‘Temporal sequence of greyscale
ultrasound changes and their relationship with neovascularity and pain in the patellar
tendon’, British Journal of Sports Medicine, 44, pp.944-947.
McCreesh, K.M., Riley, S.J. & Crotty,M. (2013) ‘Neovascularity in patellar tendinopathy and
the response to eccentric training: A case report using Power Doppler ultrasound’, Manual
Therapy,18, pp.602-605.
Ohberg, L., Lorentzon, R., Alfredson, H. (2001) ‘Neovascularisation in Achilles tendons
with painful tendinosis but not in normal tendons: An ultrasonographic investigation’, Knee
Surgery Sports Traumatology Arthroscopy, 9(4), pp.233-238.
Peace, K.A.L., Lee, J.C. & Healy, J. (2006) ‘Imaging the infrapatellar tendon in the elite
athlete’, Clinical Radiology, 61 , pp.570-578.
Peers, K. H. & Lysens, R. J. (2005) ‘Patellar tendinopathy in athletes: current diagnostic and
therapeutic recommendations’, Sports Medicine, 35, pp. 71-87.
Rio, E. Lorimer,M. Purdam,C. Samiric, T. Kidgell, D. Pearce, A, J. Jaberzadeh, S. Cook,
J.(2013) ‘The Pain of Tendinopathy: Physiological or Pathophysiological?’, Sports Medicine,
9.
Richards, P.J., Win, T., Jones, P.W. (2005) ‘The distribution of microvascular response in
Achilles tendonopathy assessed by colour and power Doppler’, Skeletal Radiology, 34,
pp.336-342
Warden, S. J. & Brunkner, P. (2003) ‘Patellar tendinopathy’, Clinics of Sports Medicine 22,
pp. 743-759.
Warden, S. J. Kiss, Z. S. Malara, F. A. Ooi, A. B. T. Cook, J. L. & Crossley, K. M. (2007)
‘Comparative Accuracy of Magnetic Resonance Imaging and Ultrasonography in Confirming
Clinically Diagnosed Patellar Tendinopathy’, The American Journal of Sports Medicine, 35,
pp. 427-436.
Zanetti, M., Metzdork, A., Kundert, H.P. (2003) ‘Achilles tendons: clinical relevance of
neovascularisation diagnosed with power doppler’, Radiology, 227,pp. 556-560.
1.Non-United ossification centres of tibial tuberosity
2.tendinopathy of patella tendons